Learning Resource
Quality Maturity Model
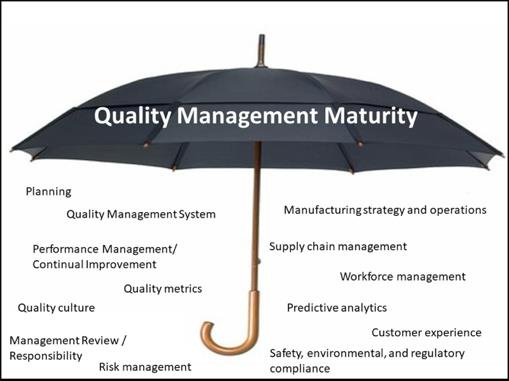
Quality Management Maturity (QMM) in pharmaceuticals is crucial as it directly impacts patient safety, regulatory compliance, and business sustainability. A mature quality management system goes beyond basic compliance, fostering a culture where quality is embedded in every process, from drug development to manufacturing and distribution. QMM helps pharmaceutical companies predict and prevent quality issues rather than simply reacting to them, leading to fewer recalls, reduced waste, and more consistent product quality. Organizations with high QMM demonstrate robust risk management, data-driven decision making, continuous improvement practices, and strong leadership commitment to quality, which ultimately results in reliable drug supply, enhanced patient trust, and competitive advantage in the highly regulated pharmaceutical market. The FDA’s emphasis on QMM ratings further underscores its importance in ensuring a stable pharmaceutical supply chain and maintaining public health standards.
Read More about FDA QMM program…
https://www.fda.gov/drugs/pharmaceutical-quality-resources/cder-quality-management-maturity
2. Continuous Manufacturing
Continuous manufacturing (CM) represents a paradigm shift from traditional batch processing, offering real-time production where materials constantly flow through an integrated system of unit operations. CM enables consistent product quality through real-time monitoring and control using Process Analytical Technology (PAT), significantly reducing manufacturing time and facility footprint. This technology allows for rapid scale-up, minimizes human intervention, and reduces the risk of batch-to-batch variability. Key advantages include reduced inventory costs, improved quality consistency, and faster response to market demands, though initial investment in equipment and training can be substantial. The FDA strongly supports CM adoption as part of pharmaceutical modernization efforts.